copper electron configuration full|complete electron configuration for gold : Manila Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for . If you guess right and cash out in time, you win. If not, you lose. Aviator is fun because it is quick and tests your ability to predict and act fast. How to Play Online? To play Aviator online using Aviator Predictor, follow these easy steps. Choose a Betting Site – Aviator is available on many betting sites. Use Aviator Predictor to pick one.
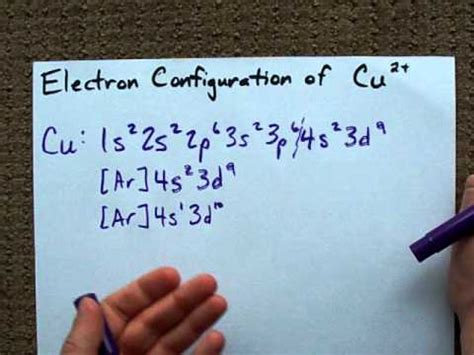
copper electron configuration full,How to Write the Electron Configuration for Copper (Cu, Cu+, and Cu2+) In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple. Full Electron Configuration For Copper. The full electron configuration for copper is [Ar] 3d10 4s1. To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of . Causey shows you step by step how to write the electron configuration and orbital notation for copper (Cu). You will learn about the orbital overlapping and the .
Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for .complete electron configuration for goldKey Takeaways. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the stability gained from a half-filled or fully-filled .
The electron configuration of copper (Cu) includes a fully-filled 3d subshell. Cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10. The actual electron configuration of .

Copper is a chemical element of the periodic table with chemical symbol Cu and atomic number 29 with an atomic weight of 63.5463 u and is classed as transition metal and is .
The electron configuration of copper is : 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Cu has a unique fully-filled 3d configuration in its ground state and so has unique physical .The full electron configuration of potassium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1; Shorthand Electron Configurations. Using potassium as an example again: . Chromium and copper have the following electron configurations, which are different to what you may expect: Cr is [Ar] 3d 5 4s 1 not [Ar] 3d 4 4s 2; To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of .Key Takeaways. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the stability gained .Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .copper electron configuration full complete electron configuration for gold From Sc on, the 3 d orbitals are actually lower in energy than the 4 s orbital, which means that electrons enter the 3 d orbitals first. In this video, we’ll discuss this in more depth and .Solution. The correct option is B 1s2,2s2p6,3s2p6d10,4s1. Cu:1s2,2s2p6,3s23p63d10,4s1. In case of copper, a completely full or half full d sub-level is more stable than a partially filled d sub-level, so an electron from the 4s orbital is excited and rises to a 3d orbital. Suggest Corrections.

Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .Unlock Full Access! Standard XII. Chemistry. Question. The electronic configuration of C u is : [N e] 3 s 2, 3 p 6, 3 d 9, 4 s 2 [N e] 3 s 2, 3 p 6, 3 d 10, 4 s 1 . Amongst the following elements whose electronic configuration are given below, the one having the highest ionisation enthaply is (a) [N e] 3 s 2 3 p 1 (b) [N e] 3 s 2 3 p 3 (c) [N .In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
copper and chromium. This order of occupation roughly represents the increasing energy level of the orbitals. Hence, electrons occupy the orbitals in such a way that the energy is kept at a . The electronic configuration of copper (Cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, which differs from the typical filling order. Copper’s 3d¹⁰ configuration in the third energy level (shell . Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator. We’re hiring! Embed. . Now use our tool as a valence electrons calculator and check the valence electrons of copper: 3 d 10 4 s 1 \rm 3d^{10} 4s^1 3 d 10 4 s 1.
copper electron configuration fullElectronic configuration of Copper: The atomic number of Copper(Cu) = 29; Therefore, the expected electronic configuration is Ar 3 d 9 4 s 2. But Copper has an exception in electronic configuration due to the stability concept of orbitals, completely filled and half filled orbitals are the most stable. Thus, the observed electronic of copper is .Copper is a chemical element of the periodic table with chemical symbol Cu and atomic number 29 with an atomic weight of 63.5463 u and is classed as a transition metal. . Full configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 1: Electrons per shell: 2, 8, 18, 1: Valence .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
The electronic configuration of copper (Cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. This configuration indicates that copper has 29 electrons distributed in its electron shells. The first shell has 2 electrons, the second shell has 8 electrons, the third shell has 18 electrons, and the fourth shell has 1 electron.
Copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure.The chemical symbol for Copper is Cu. Electron Configuration and Oxidation States of Copper. Electron configuration of Copper is [Ar] 3d10 4s1. Possible oxidation states are +1,2. Electron Configuration
copper electron configuration full|complete electron configuration for gold
PH0 · how many 3d electrons are in cu
PH1 · full electron configuration cu
PH2 · electron configuration of all elements
PH3 · electron configuration for lithium
PH4 · electron configuration explained
PH5 · electron configuration chart
PH6 · condensed electron configuration of carbon 14
PH7 · complete electron configuration for gold
PH8 · Iba pa